Workshop: Hydrogen Analysis by Atom Probe Tomography
Room 203 (large seminar room)
Max-Planck-Institut für Eisenforschung GmbH
The past decade has seen a resurgence of the interest in using atom probe tomography for the characterization of hydrogen in metals, to advance the understanding of the interaction of H with structural defects and related embrittlement phenomena.
We are organizing a workshop on 16–18 April 2024, at the Max-Planck-Institute für Eisenforschung in Düsseldorf, Germany.
With this workshop, we wanted to provide an overview of the progress in this exciting area, with advances in understanding the performance limits and how to overcome the key hurdles we are facing as a field.
We have also invited experts from related techniques – including electron microscopy, thermal desorption spectroscopy, in-situ nanoindentation, and secondary-ion mass spectrometry – to help position results from atom probe and identify the complementarities across the techniques, and evaluate how information from atom probe can be used in conjunction with existing theories to advance the understanding of the many effects that hydrogen has on the properties of materials.
We will leave ample time for discussions, in order to help define good practice across our emerging field. We will be planning to record sessions to leave a legacy, and write down a white paper to facilitate sharing good practice across the field.
We are expecting that the workshop will be free to attend (working on getting sponsorship as we write this).
We hope to see you join us in Düsseldorf!*
*We are thrilled to welcome you onsite, and for those unable to join in person, this event is also accessible in a hybrid format!
-
-
Invited talks
-
1
I knew you were trouble: analyzing hydrogen analysis by APTSpeaker: Prof. Baptiste Gault (Max-Planck-Institute für Eisenforschung GmBH)
-
1
-
Applied materials: hydrogen in solid solution
-
2
Insights into Hydrogen Embrittlement of Nickel Single Crystal Superalloys
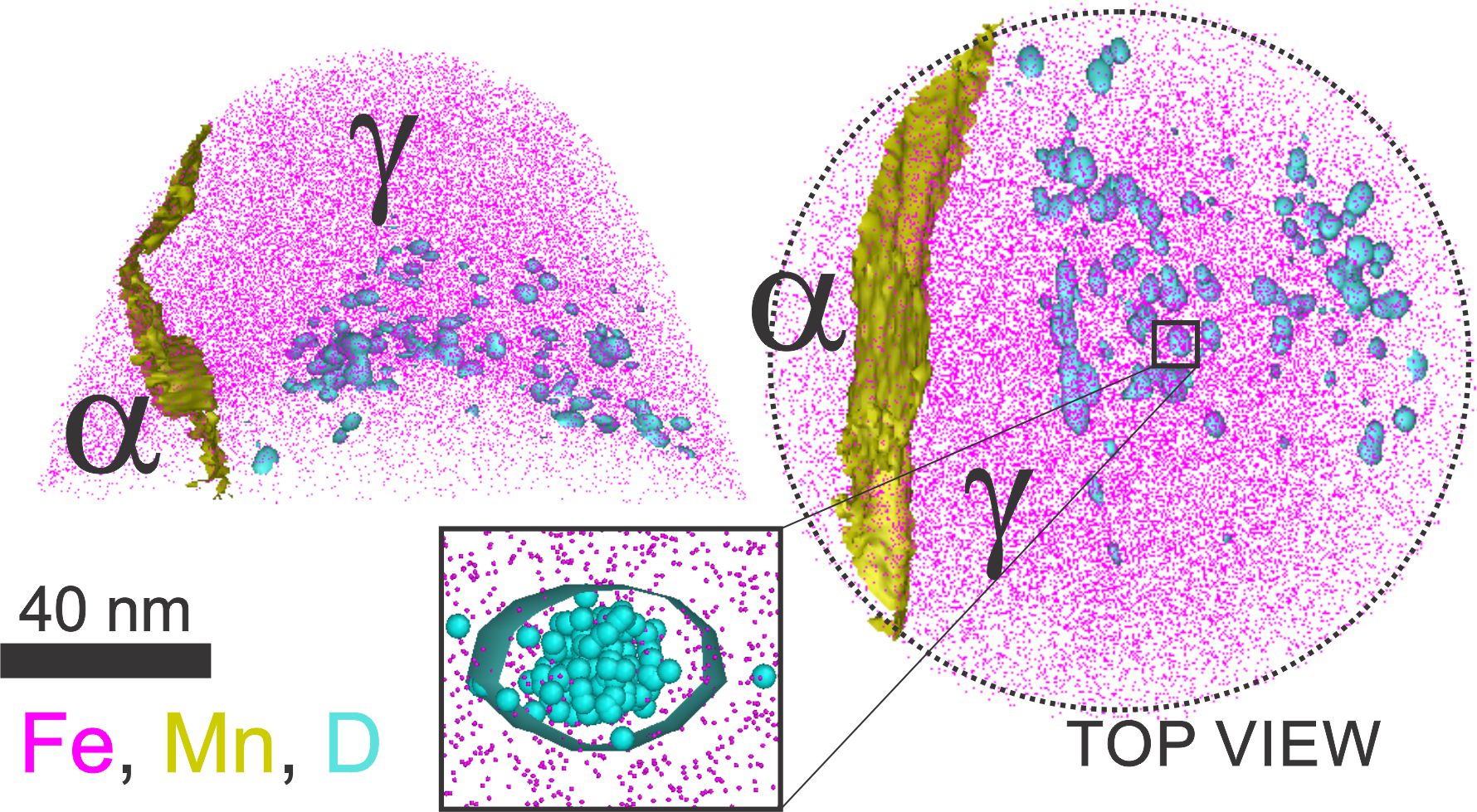
Nickel base single crystal superalloys with higher volume fraction of γˊ are known to be severely susceptible to hydrogen embrittlement. Hydrogen is found to increase the slip planarity, accumulate dislocations in γ channels causing a macroscopic fracture along {001} γ/γˊ interface [1]. There is long standing enigma on the exact reason behind this γ/γˊ interfacial failure. Few theoretical studies predict the segregation of H to γ/γˊ interface as the cause for this [2,3]. However, there is no evident experimental proof to show preferential segregation of H to any of the microstructural features. Our focus is to understand preferential segregation of H using APT with D as tracer element and correlating it with other experimental results to get better insights into the γ/γˊ interfacial failure.
Speaker: Surendra Kumar Makineni (Indian Institute of Science Bangalore 560012) -
3
Hydrogen and deuterium distributions in additively manufactured high-strength steels: Initial APT results
Hydrogen as the lightest element and the smallest atom in the periodic table tends to diffuse fast with the capability to re-distribute quickly and penetrate almost all materials. Understanding the interactions of hydrogen with host materials is of great interest for many applications, from hydrogen storage and green energy applications to hydrogen embrittlement. We use atom probe tomography (APT) to characterize atomic-scale distributions of hydrogen in an additively manufactured martensitic stainless steel. Hydrogen is essentially ubiquitous, including sample and equipment surfaces, and thus likely to cause a significant background signal in APT, we use isotopic labeling with deuterium (D) introduced via electrochemical charging to permit more detailed tracking of D and H in our samples. The local concentrations of H and D in the APT reconstructions are correlated with microstructural features present in this stainless steel, such as carbide precipitates and martensitic lath boundaries. We critically discuss the amounts and distributions of H and D detected to elucidate their potential trapping at these microstructural features and imperfections.
Speaker: Dieter Isheim (Department of Materials Science and Engineering Manager, Northwestern University Center for Atom-Probe Tomography Northwestern University)
-
2
-
10:25
Coffee Break
-
Applied materials: hydrogen in solid solution
-
4
Atomic-scale analysis of hydrogen embrittlement and corrosion in high-strength Al alloys
Abstract: Environmentally assisted embrittlement of high-strength Al alloys hinders their wide applications. The important role of hydrogen (H) associated with the H “embrittlement” mechanism occurs. However, the challenge of assessing the precise trapping sites of H makes the mechanisms remain ambiguous. In this presentation, I discuss the findings on H associated with specific microstructural features in a high-strength 7xxx Al alloy [1] investigated using atom probe tomography. We successfully achieved visualization and assessment of H at second-phases and grain boundaries, with the enrichment of one order of magnitude higher as opposed to the Al matrix. We used these observations to guide atomistic ab initio calculations, which show that the co-segregation of alloying elements and H favours grain boundary decohesion, and the strong partitioning of H into the second-phase particles removes solute H from the matrix, hence preventing H embrittlement. In the second part [2], I talk about the recent results regarding direct evidence that the oxide acts as a trap for H, pointing at the essential role of the Al oxide might act as a kinetic barrier in preventing H embrittlement. These insights further advance the mechanistic understanding of H-assisted embrittlement in Al alloys, emphasizing the role of H traps in minimizing cracking and guiding new alloy design.
Keywords: Hydrogen embrittlement, 7xxx Al alloy, Mechanical properties, Atom probe tomography, Ab-initio calculations
References:
[1]. H. Zhao, P. Chakraborty, D. Ponge, T. Hickel, B. Sun, C. Wu, B. Gault, D. Raabe, Hydrogen trapping and embrittlement in high-strength Al alloys. Nature 602, 437-441, (2022).
[2]. H. Zhao, Y. Yin, Y. Wu, S. Zhang, A. Mingers, D. Ponge, B. Gault, M. Rohwerder, D. Raabe, How solute atoms control aqueous corrosion of Al alloys, Nature Communications, 15, 561, (2024).Speaker: HUAN Zhao (Xi'an Jiaotong University)
-
4
-
Invited talks
-
5
Controlled boron segregation in martensitic steels to improve the resistance against hydrogen embrittlement
Hydrogen is nowadays viewed as a key element for developing low-carbon energies, but it diffuses in metals and alloys and segregates in crystalline defects, reducing the ductility of the material. This phenomenon is well-known and called hydrogen embrittlement (HE). In this work, we aim to reduce the negative impact of hydrogen by adding boron at interfaces to improve the resistance against HE.
Boron mostly segregates into prior austenite grain boundaries (PAGBs) before the martensitic transformation in steels. This segregation naturally improves resistance to HE compared to boron-free steels by avoiding the intergranular fracture at PAGBs [1]. The competition between boron and hydrogen at PAGBs has been investigated further using thermal desorption spectroscopy (TDS) experiments and ab initio calculations. The latter shows that even if the interaction between hydrogen and the structural defect is attractive, it becomes repulsive when boron is present in the grain boundary. This behaviour has also been observed using TDS measurements, with the disappearance of one peak when boron is incorporated into the microstructure.
The hydrogen concentration partitioned in the microstructure has then been quantified from peak integration of the TDS measurements and compared to the hydrogen concentration at thermodynamic equilibrium. The latter concentration has been evaluated using the Langmuir-McLean approximation with trapping energy deduced from the TDS measurements and permeation tests. This thermodynamic model shows that all traps are filled identically when the total hydrogen concentration is low for boron-free steel. However, when it increases, traps of the lowest segregation energies (mostly PAGBs) are firstly saturated, which promotes failure initiation at this defect type. This finding partially explains why PAGBs are the weakest microstructure feature when martensitic steels are exposed to hydrogen-containing environments.
However, HE is still observed when the hydrogen content is increased due to a fracture at martensite boundaries, interfaces where boron is not located [1]. Additional heat treatments have been performed to activate the mobility of boron and carbon in the solid solution which can segregate in other martensitic interfaces (packets, blocks, and lath boundaries). This heat treatment improves the resistance of boron-doped steels to avoid a cleavage fracture by reducing the hydrogen uptake. The newly developed microstructure is analysed through synchrotron experiments and atom probe tomography, which confirms the segregation of boron at interfaces without any change in the stress states (dislocation density and crystalline size).
References
[1] H. Shi, G. Hachet, P.T. Sukumar, D. Ponge, B. Sun and D. Raabe, Internal communication, 2024
[2] G. Hachet, A. Tehranchi, H. Shi, M. Prabhakar, D. Ponge and D. Raabe, Communication submitted, 2023Speaker: Guillaume Hachet (Max-Planck-Institut für Eisenforschung)
-
5
-
Background and instrumentation
-
6
Background knowledge about hydrogen behaviour in the context of field ion emitters
This presentation provides a reminder of our existing background knowledge about how hydrogen behaves in the context of field ion emitters. This may or may not prove to be of detailed relevance to the analysis of hydrogen-related atom-probe data, but it would probably be useful to have it available as background information. The following behavioural effects will be noted.
(1) The likely dynamic behaviour of any hydrogen molecules desorbed from the inner surface of a metal vacuum chamber. This will be similar to the behaviour of the operating gas in a field ion microscope or gas field ion source, which has been summarised before [1].
(2) The propensity for hydrogen molecules to dissociate into hydrogen atoms on reaching a metal surface [2].
(3) The phenomenon of "hydrogen promoted field ion imaging". In my view, the physical origin of this effect is still not clearly established (see [3] for a 1974 discussion). The issues are what form the hydrogen takes and precisely where it is located on the emitter surface. The relevance to this workshop is the question: "What happens to an internal hydrogen entity, as field evaporation moves the emitter surface back towards it?
(4) The possibility that what is field evaporated is a complex between a hydrogen molecule or atom and an atom of the material substrate.
(5) The possibility that a field-evaporated complex may break-up in flight.
Attention will also be briefly drawn to three aspects of conventional atom-probe theory where small improvements could possibly/probably be made, as follows.
(6) Better understanding of the full physics behind the shape of a field-evaporated endform.
(7) Better understanding of the theory behind the experimental fact that radial distance in field electron, field ion and atom-probe images is proportional to "crystallographic angle" between the local surface normal and the emitter axis.
(8) Better understanding of the relationship between the parameter called "image compression factor" in atom-probe data analysis and the various different parameters called "angular magnification" in the context of charged-particle optics as understood outside the atom-probe community.
Attention is also drawn to a recent topical review [4] of field emitter electrostatics, including reference to highly efficient numerical simulation procedures. This review applies to both field electron and field ion emitters, although the main focus is field electron emission. There is probably a need to correlate this with recent developments in field ion emitter electrostatics, as used for example in [5].[1] R.G. Forbes, Appl. Surface. Sci. 94/95, 1 (1996).
[2] Carry out a websearch on "hydrogen dissociation at metal surfaces" to discover many relevant papers.
[3] T. Sakurai, T.T. Tsong, and E.W. Müller, Phys. Rev. B 10, 4205 (1974).
[4] T.A. de Assis, F.F. Dall'Agnol and R.G. Forbes. J. Phys.: Condens. Matter 34, 493001 (2022).
[5] C. Hatzoglou et al., Microsc. Microanal. 19, 1124 (2023).Speaker: Richard G. Forbes -
7
Experimental approaches to maximise accurate APT hydrogen-quantification in metallurgical materials
Across a wide-range of applications in materials science, there is growing interest in being able to accurately detect, locate and quantify the presence of hydrogen in the microstructures of materials. These include modern high-strength steels, Ni-alloys and Ti-alloys for aerospace and other engineering applications, broadly aiming to understand how to make components more resistant to the embrittling effects of hydrogen. In a similar vein, a wide array of metallic surfaces exposed to extreme environmental conditions of irradiation and high temperatures in nuclear applications (both fission and fusion processes) can see hydrogen ingress, and understanding the extent and rate of this is crucial to predicting component lifespans.
While APT instrumentation can routinely detect hydrogen in various forms, accurately quantifying this is uniquely prone to difficulties decomposing any true signals from levels of contaminant or background hydrogen inside the atom probe vacuum chamber. We have developed a number of approaches to help tackle this problem, such as using deuterium (D+) ions as a proxy for hydrogen in a variety of different experimental charging protocols. This has been done in parallel with concerted efforts to both determine the likely level of expected background hydrogen peaks and to minimize these by systematically varying the instrument analysis conditions. A number of such studies will be presented along with suggested strategies for future experiments in this area.[1] M. S. Meier, M. E. Jones, P. J. Felfer, M. P. Moody, and D. Haley, “Extending Estimating Hydrogen Content in Atom Probe Tomography Experiments Where H2 Molecule Formation Occurs,” Microsc. Microanal., vol. 28, no. 4, pp. 1231–1244, Aug. 2022, doi: 10.1017/S1431927621012332.
[2] M. S. Meier, P. A. J. Bagot, M. P. Moody, and D. Haley, “Large-Scale Atom Probe Tomography Data Mining: Methods and Application to Inform Hydrogen Behavior,” Microsc. Microanal., vol. 29, no. 3, pp. 879–889, Jun. 2023, doi: 10.1093/micmic/ozad027.Speaker: Paul A. J. Bagot (St. Catherine's College Lecturer, University of Oxford, Department of Materials)
-
6
-
12:30
Lunch
-
Poster
-
8
Micro- to atomic-scale characterisation of hydrogen ingress at the oxide/metal interface of nuclear Zr-alloys
Zr alloys have been broadly used as cladding materials in fission reactors since 1950s [1, 2]. A universal degradation issue in this field is the oxidation of Zr cladding materials, which has been widely investigated in the past decades [3-5], but there is insufficient understanding of hydrogen ingress process during corrosion due to the fundamental difficulties in hydrogen detection. Atom probe topography (APT) has an equal and very high detection sensitivity to all elements [6], but contaminant H is readily and frequently incorporated during tip preparation using focussed ion beam (FIB) and from the adsorption of H2 from the analysis chamber metal walls. It is thus challenging to distinguish the contaminant H and inherent H within materials from the 1 Da and 2 Da peaks in the mass spectrum, making H analysis using APT very complicated and prone to major quantification uncertainties. This situation can be mitigated by introducing deuterium (D) in the form of D2O (heavy water), where D atoms appear at the 2 Da peak as D+ and 3 Da peak as DH+.
In this study, low-tin ZIRLO® [7] samples subjected to autoclave tests with H2O water for the first 10 days and 50% D2O water for the following 20 days were characterised by APT, nanoscale secondary ion mass spectrometry (NanoSIMS), transmission electron microscopy (TEM), and transmission Kikuchi diffraction (TKD) at the oxide/metal interface. The presence of D and supporting data from other techniques assist to better interpret the APT hydrogen data, and the novel insights of hydrogen ingress process can be utilised to understand the whole story of the oxidation process.
Reference:
1. Krishnan, R. and M.K. Asundi, Zirconium alloys in nuclear technology. Proceedings of the Indian Academy of Sciences Section C: Engineering Sciences, 1981. 4(1): p. 41-56.
2. Lemaignan, C. and A.T. Motta, Zirconium Alloys in Nuclear Applications, in Materials Science and Technology. 2006.
3. Lemaignan, C., Corrosion of Zirconium Alloy Components in Light Water Reactors. Vol. 13C. 2006, ASM International. 415-420.
4. Couet, A., A.T. Motta, and A. Ambard, The coupled current charge compensation model for zirconium alloy fuel cladding oxidation: I. Parabolic oxidation of zirconium alloys. Corrosion Science, 2015. 100: p. 73-84.
5. Porte, H.A., et al., Oxidation of Zirconium and Zirconium Alloys. Journal of The Electrochemical Society, 1960. 107(6): p. 506.
6. Meier, M.S., et al., Exploiting Adsorption Dynamics in Atom Probe Tomography for accurate Measurements of Hydrogen Concentrations. Microscopy and Microanalysis, 2022. 28(S1): p. 1650-1652.
7. Sabol, G.P., ZIRLO™ — An Alloy Development Success, in Zirconium in the Nuclear Industry: Fourteenth International Symposium, B. Kammenzind, Editor. 2005, ASTM International: West Conshohocken, PA. p. 3-24.Speaker: Mr Wenyu Zhang (Department of Materials, University of Oxford) -
9
Ultra-lightweight compositionally complex alloys with large ambient-temperature hydrogen storage capacity
In the burgeoning field of hydrogen energy, compositionally complex alloys promise unprecedented solid-state hydrogen storage applications. However, compositionally complex alloys are facing one main challenge: reducing alloy density and increasing hydrogen storage capacity. Here, we report TiMgLi-based compositionally complex alloys with ultralow alloy density and significant room-temperature hydrogen storage capacity. The record-low alloy density (2.83 g cm-3) is made possible by multi-principal-lightweight element alloying. Introducing multiple phases instead of a single phase facilitates obtaining a large hydrogen storage capacity (2.62 wt.% at 50 °C under 100 bar of H2). The kinetic modeling results indicate that three-dimensional diffusion governs the hydrogenation reaction of the current compositionally complex alloys at 50 °C. The here proposed approach broadens the horizon for designing lightweight compositionally complex alloys for hydrogen storage purposes.
Speaker: Yuanyuan Shang (Helmholtz-Zentrum hereon GmbH) -
10
Hydrogen Trapping in Transition Iron Carbides in Martensitic Advanced High Strength Steels
Martensitic advanced high strength steel (MS-AHSS), specifically MS1500, is an identified steel for various applications in the automotive industry to reduce the car weight improving fuel economy without compromising structural integrity. Nevertheless, hydrogen embrittlement (HE) is an issue with MS-AHSS. The HE susceptibility is affected by a number of the steel’s characteristics including the strength of the material, microstructure, tempering temperature, and precipitates. While the microstructure of MS1500 is considered to be “fully martensitic”, TEM and atom probe tomography have revealed nanoscale iron carbide precipitates that have transformed within martensitic lathes. These nanoscale precipitates have been identified to be the carbon ordering Fe4C phase. These steels were then hydrogen charged by electrolysis revealing that these carbides act as traps. This paper will address the experimental details of hydrogen charging and postulated geometric rationales why the Fe4C can trap the hydrogen within its interior rather than other carbides, which report trapping at the interface of carbide and steel matrix.
Speaker: Madyson Canulette (The University of Alabama) -
11
Enhancing grain boundary cohesion by B-doping mitigates H-induced intergranular failureSpeaker: Mohamed Elkot (Max-Planck-Institut für Eisenforschung GmbH)
-
12
The unexplored behavior of D/H at semi-coherent austenite-ferrite phase boundariesSpeaker: Ms Xizhen Dong (Max-Planck-Institut für Eisenforschung GmbH)
-
13
Electron irradiation-caused hydrogen segregation on Al-Mg alloy grain boundariesSpeaker: Dr Xinren Chen (Max-Planck-Institut für Eisenforschung GmbH)
-
14
The mechanism and challenges of hydrogen detection with SKP and SKPFMSpeaker: Mr Bilgehan Murat Sesen (Max-Planck-Institut für Eisenforschung GmbH)
-
15
Role of diffusive hydrogen on the mechanical behavior of BCC FeCr alloys by in situ nanoindentationSpeaker: Dr Jing Rao (Max-Planck-Institut für Eisenforschung GmbH)
-
8
-
-
-
Invited talks
-
16
Mapping hydrogen activity at high local resolution by scanning Kelvin probe techniques
Scanning Kelvin Probe (SKP) techniques have now been in use for mapping hydrogen in materials for about a decade. Different from techniques such as Secondary Ion Mass Spectroscopy (SIMS) or Atom Probe Tomography (APT) they do not measure the hydrogen concentration at a certain site, but rather the local activity. In time-resolved experiments they can provide information about how much hydrogen can be released from a certain trap site and at what rates. However, although many successful applications are reported up to now, still a number of challenges have to be overcome. For instance, quantification requires the use of a suitable detection layer that, however, can be quickly saturated at high activities. Furthermore, especially very small microstructures are often a challenge, as background hydrogen can blanket the local hydrogen. Examples will be presented as well as strategies for how to overcome problems met.
Speaker: Michael Rohwerder (Max-Planck-Institut für Eisenforschung)
-
16
-
Background and instrumentation
-
17
Pulsed laser experiments in an ultra-low H atom probe
While atom probe experiments to detect hydrogen are not new, special measures had to be taken to distinguish the H detected from the specimen from the contaminant unless molecular ion formation enabled identification. This is largely due to the residual hydrogen in conventional stainless steel atom probes. The distinction was mostly made by isotope, using deuterium or recently even tritium as tracers. This somewhat limits the scope of the experiments, but has certainly been a practical approach. However, especially for imaging, the tracer approach demands voltage pulsed APT experiments, since in laser pulsed experiments the formation of molecular H species renders interpretation of ionic identity from mass-to-charge very difficult. Many hydrogen embrittled materials however only yield relatively little data when using voltage pulsing.
In order to enable pulsed laser experiments to detect H, we have equipped our ultra-low H titanium atom probe with pulsed laser, with IR (1064), green (532) and DUV (266) wavelengths at a pulse duration of 400 fs and an unpicked pulse repetition rate of 400 kHz. The authors have already demonstrated that this instrument has a consitant improvement of H background of at least two orders of magnitude over conventional APT systems with stainless steel chambers, but was so far lacking a laser. This laser has now been installed and the optics are being built up. At the workshop we will present the status of the laser setup and some first experiments. These will hopefully show if the major improvement this instrument has brought in pulsed voltage mode can also be transferred to pulsed laser experiments.Speaker: Peter Felfer (Friedrich-Alexander Universität Erlangen-Nürnberg) -
18
Exploring measurement parameters and modes of the newest generation of LEAP instruments for quantification of hydrogen
So far, the most common practice for measuring H at microstructural features in APT is using the heavier isotope deuterium in combination with voltage pulses. For metals with a high evaporation field, including steels, the formation of H2+ ions is inhibited. Hence, the peak at 2 Da can solely be attributed to D, which was introduced via electrochemical or gas charging, or implantation. But what about samples, that fracture easily in voltage mode? Usually, laser-assisted evaporation is employed for these materials, which incurs a higher concentration of H and the formation of H2+. The amount of artificial H from the vacuum chamber can be estimated by the evaporation rate method, i.e. varying the time between pulses.
In this work, commonly used measurement parameters like constant detection rate or frequency are compared with measurements with constant flux of ions and varying frequencies. The newest generation of LEAP instruments is equipped to run simultaneous voltage and laser pulses. This feature allows the use of low laser energy while keeping a low field between pulses to limit background noise. In this way, H2+ formation is mitigated, while ensuring smooth evaporation of the samples. The concentration of H is tracked throughout the experiments and visualized as a function of time between pulses. Measurement parameters to distinguish artificial H2+ from introduced D+ at the 2 Da peak are investigated.Speaker: Severin Jakob (Chalmers University of Technology)
-
17
-
10:25
Coffee Break
-
Invited talks
-
19
Strategy development for measurements of hydrogen in APT and environmental TEM
Hydrogen as the smallest atom of all elements requires special care when one wants to detect it. Because of its presence in the background gas of any UHV-system, its capability to propagate in materials by quantum mechanical tunnelling and the presence of clean surfaces during the APT analysis of the nano-sized tips are challenging tasks to address. Especially the quantitative measurement is tricky, when hydrogen concentrations beside that of hydrides are of interest. We designed a strategy to quantitatively determine the hydrogen content in the voltage pulsed – APT. This development will be addressed and verified on multilayer samples. EELS and microstructural changes allow the localisation of hydrides in the TEM. Environmental cells or ETEMs enable to studying the hydride formation in-situ, in the presence of hydrogen gas. This will be demonstrated on the hydriding process in Mg-films.
Speaker: Prof. Astrid Pundt (KIT)
-
19
-
Hydrogen charging via unconventional methods
-
20
APT of Deuterium in tungsten for fusion applications
W is a candidate material for plasma-facing components but is also being considered as a liquid metal corrosion barrier in the breeder blanket of future fusion devices. In both scenarios, the W will be exposed to tritium. Radioactive tritium inventory must be controlled to ensure safe, fuel-efficient powerplant operation; it is essential to understand hydrogen isotope trapping and transport in W.
Deuterium inventory can be quantified using techniques such as thermal desorption spectroscopy (TDS), and information about the types of deuterium trap sites present can be obtained from the TDS spectra through complementary first principles modelling. However, spatial mapping of hydrogen isotopes on length-scales comparable to nanoscale trap sites is challenging since it is difficult to detect low hydrogen concentrations and resolve hydrogen isotopes with most established microscopy techniques.
This work uses atom probe tomography (APT) to map deuterium within ion irradiated and unirradiated W on the nanoscale. Deuterium was introduced through exposure of bulk samples to low-energy deuterium plasma. APT-measured deuterium depth profiles show co-location of deuterium and oxygen. C/O/N clustering is observed, but with no deuterium co-segregation.
Deuterium-grain boundary interactions are revealed in the atom maps of the fine-grained, unirradiated W sample. Experimental observations are complemented by first principles modelling to understand the role of irradiation-induced and as-received defects in trapping and transport of hydrogen isotopes in W and W oxides.Speaker: Hazel Gardner (UKAEA) -
21
In-situ Hydrogen Implantation in Atom Probe Tomography and Investigation of Hydrogen Embrittlement
The investigation of hydrogen in Atom Probe Tomography remains a relevant challenge. Its low mass, high diffusion coefficient, and presence as a residual gas in vacuum chambers generate multiple complications for APT investigations. Different solutions were proposed in the literature to charge our sample, such as ex-situ charging coupled with cryotransfer [1], or hydrogen charging at high temperatures in a separate chamber [2]. Nevertheless, these solutions often faced challenges due to the complex control of specimen temperature during hydrogen charging and subsequent analysis.
In this paper, we propose an alternative route for in-situ H charging in atom probe derived from a method developed in field ion microscopy to study He implantation damage. By applying negative voltage nanosecond pulse on the specimen in an atom probe chamber under low pressure of H2, it is demonstrated that high dose of H (~100,000 at) can be implanted in the range 2-20 nm beneath the specimen surface. An atom probe chamber was modified to enable direct negative pulse application with controlled gas pressure, pulse repetition rate and pulse amplitude. Through electrodynamical simulations, we show that the implantation energy falls within the range 100 - 1,000 eV. A theoretical depth of implantation was predicted and compared to results.
To investigate the hydrogen embrittlement, our objective is to understand the impact of hydrogen implanted within the sample by examining the evaporation electric field. A comparison is made between hydrogen coming from the analysis chamber and from the materials. The result indicates that hydrogen from the sample significantly reduces the evaporation electric field (~6%).References:
[1] Yi-Sheng Chen et al. Cryo Atom Probe: Freezing atoms in place for 3D mapping. Nano Today 37 (2021).
[2] J. Takahashi et al. Origin of hydrogen trapping site in vanadium carbide precipitation strengthening steel. Acta Materialia 153, p 193-204 (2018).Speaker: Mr Jean-Baptiste Maillet (Université de Rouen, GPM) -
22
Gas exposure devices for atom probe experiments
The detection of hydrogen in atom probe tomography (APT) tips is challenging due to the residual hydrogen in the currently used stainless steel measurement chambers. While adjusting measurement parameters can reduce the presence of contaminant hydrogen, complete elimination is not feasible and interpretability is therefore limited. Consequently, Deuterium or Tritium is employed as an alternative for investigating hydrogen in APT-tips through identification by isotopic nature. Charging of D and T can occur through either electrochemical or gas-phase methods. Gas-phase charging setups have been in use for a while and have shown promising results as they do not introduce corrosion or contamination on the specimen. The use of a gas instead of an electrolyte also removes the temperature restrictions of an aqueous electrolyte, thus enabling bulk charging of fcc materials such as austenitic stainless steels or Ni bases superalloys. Gas charging setups have been built as standalone devices or integrated into existing atom probes. However, previously existing configurations have been relatively complex and thus expensive to acquire and limited in ultimate pressure. In this presentation, we aim to showcase two approaches for hydrogen gas charging of APT-tips. One with minimal material costs and straightforward manufacturing methods, making them accessible to a broader range of laboratories, and another one with 1000 bar / 300 °C capability, requiring a dedicated H enables laboratory. The former setup is user-friendly and, due to their size, maximum temperature, and pressure application, are not subject to safety regulations. The successful use of these setups will be demonstrated through charged palladium tips. The intended use of these setups is limited to lower charging pressures (up to 10 bar at 90 °C), still allowing for a wide range of experiments with H or potentially other gases. We also introduce a system for high-pressure charging of APT-tips. This setup can generate pressures up to 1000 bar at 300 °C, enabling the charging of various types of APT-tips to levels comparable to electrochemical charging, all while introducing no contamination or corrosion.
Speaker: Benedict Ott
-
20
-
12:30
Lunch
-
Poster
-
Invited talks
-
23
Micromechanical testing during hydrogen charging
Understanding the effects of hydrogen in materials became a pressing topic with the imminent shift towards green technologies and the adoption of hydrogen as energy carrier. It is expected that the use of hydrogen will increase in all industries, together with the need for safe transport and storage and consequently the development of new materials and technologies to cope with it. A critical challenge is hydrogen-induced damage, or hydrogen embrittlement, that can cause the sudden failure of a material.
Current studies on hydrogen effects are in their majority limited to post-mortem probes and ex-situ charging, which neglect diffusible hydrogen and its migration and desorption at the analysis time. Additionally, it is necessary to characterize hydrogen and its effects in materials at the relevant small-scale dimensions where embrittlement initiates. To combine these substantial yet demanding tasks, we designed a novel “back-side” charging approach, to perform micromechanical testing during hydrogen charging. Hydrogen is generated electrochemically at the back-side and diffuses towards the testing (front-side) surface. This unique method allows differentiating between the effects of trapped and mobile hydrogen, and performing well controlled measurements with different hydrogen levels monitored over time to consider hydrogen absorption, diffusion and release.
In this talk, I will present first an overview of the technique, including its advantages and limitations. Secondly, I will discuss the effects of diffusible hydrogen on binary Fe-based alloys, unraveling the dynamic effects of hydrogen using time-resolved nanoindentation testing. Finally, I will introduce the application of the method to study Al2O3 as a hydrogen barrier coating, which is an appealing option to prevent and/or slow down the hydrogen ingress into structural alloys that are susceptible to embrittlement. The mechanical testing is always complemented by additional techniques to measure the hydrogen behavior, such as thermal desorption spectroscopy and Kelvin probe testing, and the changes in the microstructure, including scanning and transmission electron microscopy, and atom probe tomography. Particularly, I will highlight the relevance of this last technique, where more complementary studies are required.Speaker: Dr Maria Jazmin Duarte (Max-Planck-Institut für Eisenforschung GmbH)
-
23
-
Hydrogen charging via unconventional methods
-
24
Gas phase deuterium charging of polymer nanocomposites for APT analysis
APT analysis of polymers has been relatively limited compared to many other material systems due to sample preparation, data collection and data analysis challenges. Polymers are often beam sensitive and Focused Ion Beam (FIB) sample preparation is often avoided, leading to approaches based on spray deposition of polymer films [1] or self assembling monolayers [2] onto pre-fabricated sharpened supports. Mass spectra collected from polymers can consist of carbon compound ions, and halogens used in polymers such as chlorine or fluorine are difficult to ionise and so compositional analysis can also be challenging [3].
Boron nitride 2D nanoparticles (NP) within a thermoplastic semi-crystalline polymer matrix (polyvinylidene fluoride (PVDF)), so called polymer nanocomposites (PNC) – are systems considered as promising material candidates for gas barrier applications. PNC samples were prepared using a melt-compounding process at different NP loadings, injection moulded into discs and characterised using thermogravimetric analysis, differential scanning calorimetry and scanning electron microscopy, alongside gas permeation studies at the macroscopic level.
Laser pulsed analysis using a LEAP 5000XR of these samples by the authors produced a mass spectra that contains various compound ions linked to fragments of the polymer chain. In the samples predicted to contain nanoparticles (visible through secondary electron imaging during Focused Ion Beam sample preparation), localised regions that contained boron species were observed along with various compound ions of carbon, boron and fluorine. Hydrogen species were found in many locations within the reconstruction and so in order to determine their origin, gas phase deuteration is required.
In this presentation we discuss the workflows and challenges for deuterium charging and APT analysis of polymer nanocomposites and initial results collected using the Reacthub facilties at Max-Planck-Institut für Eisenforschung.[1] Journal of Microscopy, 2010, 237,2 pp. 155–167
[2] Langmuir 2010, 26, 8, 5291–5294.
[3] Langmuir 2012, 28, 1, 56–59.Speaker: James Douglas (Imperial College London) -
25
Interaction of Hydrogen/ Deuterium with defects in Dual Phase Steels
Hydrogen (H) is the most abundant element in the Universe. It has a great potential to replace the C-based fuels as a more sustainable energy solution. In order to support and implement this transition towards a more sustainable H economy, understaning the interaction of H with its surrounding infrastructure requires immediate attention. H embrittlement (HE) is a phenomenon that causes abrupt loss in the load bearing capacity of large engineering structures in the presence of H. Its limited understanding poses a hurdle for the transition to a H based economy. High strength steels are particularly prone to HE where even less than 1 part per million by weight (ppmw) H is sufficient to dramatically degrade the mechanical properties [1]
Medium Mn steels consisting of a dual-phase, austenite-ferrite microstructure have been employed for its enhanced transformation induced plasticity (TRIP) effect to achieve an improved strength-ductility combination [2]. However, its multiphase microstructure with difference in H solubilities and diffusivities makes HE studies on this material system challenging. A recent study elucidated to the presence of strong H trapping sites with an activation energy of up to 50 kJ/mol in such steels, hinting towards H trapping at the austenite-ferrite phase boundaries (PBs) [3].
Here, a medium Mn steel (0.2C-10Mn-3Al-1Si) is heat treated to produce a microstructure with a high density of PBs which will enable us to bypass the need for site specific specimen preparation for APT. Additionally, the microstructure revealed an approximately equal fraction of semi-coherent and incoherent PBs. We investigate and visualize interaction of H by systematically probing vacancy clusters, dislocations, and austenite-ferrite PBs via atom probe tomography (APT).
References
[1] B. Sun, W. Lu, B. Gault, R. Ding, S.K. Makineni, D. Wan, C.-H. Wu, H. Chen, D. Ponge, D. Raabe, Chemical heterogeneity enhances hydrogen resistance in high-strength steels, Nature Materials (2021).
[2] B. Sun, Y. Ma, N. Vanderesse, R.S. Varanasi, W. Song, P. Bocher, D. Ponge, D. Raabe, Macroscopic to nanoscopic in situ investigation on yielding mechanisms in ultrafine grained medium Mn steels: Role of the austenite-ferrite interface, Acta Materialia 178 (2019) 10-25.
[3] B. Sun, W. Krieger, M. Rohwerder, D. Ponge, D. Raabe, Dependence of hydrogen embrittlement mechanisms on microstructure-driven hydrogen distribution in medium Mn steels, Acta Materialia 183 (2020) 313-328Speaker: Dr Aparna Saksena
-
24
-
Establishing best practices when measuring hydrogen in APT (a structured discussion)
-
-
-
Invited talks
-
26
Hydrogen Localisation in Metallurgical Samples with High Resolution Secondary Ion Mass Spectrometry (NanoSIMS)
The NanoSIMS is emerging as a powerful tool to study complex problems in materials science and, along with atom probe tomography, is one of the few techniques able to localise hydrogen and deuterium at microstructurally relevant length scales. The NanoSIMS is a high-resolution secondary ion mass spectrometry instrument capable of chemical mapping at <100 nm spatial resolution, detection limits in the ppm range and is able to detect almost all elements in the periodic table as well as isotopes. It uses a 16 kV Cs+ ion probe to generate secondary ions from the sample surface which are collected and analysed in a magnetic mass spectrometer. The NanoSIMS has seven detectors allowing simultaneous detection of different elements and isotopes along with an ion-induced secondary electron image giving topographical information.
In this presentation I will show how we have been using the NanoSIMS to localise deuterium in electrochemically charged steel and nickel alloys as well as in zirconium alloys oxidised in an autoclave to simulate nuclear reactor conditions. I will explain the requirement to use deuterium to minimise imaging artefacts and uncertainties in the origin of the detected hydrogen. The complexities associated with detecting hydrogen and deuterium in the NanoSIMS will be discussed and I will also show how NanoSIMS compares to the information that can be obtained with atom probe tomography in terms of spatial resolution and volume of material analysed.
Speaker: Katie Moore (University of Manchester)
-
26
-
Hydride forming materials
-
27
Resolving hydrogen atoms in metal hydrides using scanning transmission electron microscopy
Hydrogen as a fuel can be stored safely with high volumetric density in metals. It can, however, also cause embrittlement of metals. Understanding fundamental behavior of hydrogen at atomic scale is key to improve metal – metal hydride systems. However, currently, it is challenging to visualize hydrogen atoms. Here, we present our recent work in which we imaged for the first time hydrogen atoms at a metal – metal hydride interface and demonstrated a new techniques allowing robust imaging of hydrogen in metal hydrides [1]. The new technique is called integrated differential phase contrast, a recently developed technique for scanning transmission electron microscopy. To demonstrate the capabilities of the technique we studied titanium monohydride that forms thin and long plates inside titanium. The titanium – titanium monohydride interfaces are coherent, but large stresses are present perpendicular to the interfaces explaining the extreme aspect ratio of the plates and why they cause embrittlement of titanium. Images of the interface reveal remarkable stability of the hydride phase, originating from the interplay between interface coherence and stresses. We also uncovered, thirty years after three models were proposed, which one describes the position of the hydrogen atoms at the interface. Our work enables novel research on hydrides and is extendable to all materials containing combinations of light and heavy elements.
[1] Sytze de Graaf, Jamo Momand, Christoph Mitterbauer, Sorin Lazar, Bart J Kooi, Resolving hydrogen atoms at metal-metal hydride interfaces, Science Advances 6 (5), eaay4312, 2020, (DOI: 10.1126/sciadv.aay4312)Speaker: Bart J. Kooi (Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands) -
28
Combining Sample Preparation, Data Analysis, and Computational Modelling to Better Understand Hydrogen Behaviour in Zirconium Alloys
Understanding the behaviour of hydrogen in zirconium-based alloys is desired since these alloys are used for fuel cladding in light water nuclear reactors but are known to suffer from hydrogen pick-up and embrittlement during service, and delayed hydride cracking (DHC) during storage.
Reliably and accurately imaging hydrogen with atom probe tomography (APT) is challenging due to several phenomena that can lead to an artificial increase in hydrogen signal during an APT experiment[1,2]. With certain materials, such as zirconium, there is the added complication that common sample preparation routes can introduce additional hydrogen into specimens and even lead to phase transformations, altering the microstructure prior to analysis[3].
In this talk, we build on the work of Hanlon et al.[3] and Mayweg et al.[4] to show how the hydrogen levels present in zirconium samples vary in specimens prepared using Ga+ and Xe+ focused ion beams (FIB) at ambient and cryogenic temperatures. Transmission electron micrographs will be shown alongside APT data and estimates will be made as to the amount of hydrogen present in APT needles after FIB preparation.
We will then present results from a recent article[5], discussing how the use of cryogenic FIB preparation of zirconium samples can be combined with careful data analysis and computational modelling to enhance our understanding of hydrogen behaviour within the microstructure of zirconium alloys.
References
1. Yoo, S. H. et al. Origins of the hydrogen signal in atom probe tomography: Case studies of alkali and noble metals. New Journal of Physics 24, (2022).
2. Sundell, G., Thuvander, M. & Andrén, H. O. Hydrogen analysis in APT: Methods to control adsorption and dissociation of H2. Ultramicroscopy 132, 285–289 (2013).
3. Hanlon, S. M., Persaud, S. Y., Long, F., Korinek, A. & Daymond, M. R. A solution to FIB induced artefact hydrides in Zr alloys. Journal of Nuclear Materials 515, 122–134 (2019).
4. Mayweg, D., Eriksson, J., Bäcke, O., Breen, A. J. & Thuvander, M. Focused Ion Beam induced hydride formation does not affect Fe, Ni, Cr-clusters in irradiated Zircaloy-2. Journal of Nuclear Materials 581, 154444 (2023).
5. Jenkins, B. M. et al. Experimental and modelling evidence for hydrogen trapping at a β-Nb second phase particle and Nb-rich nanoclusters in neutron-irradiated low Sn ZIRLO. Journal of Nuclear Materials 587, 154755 (2023).Speaker: Benjamin Jenkins (Université de Rouen)
-
27
-
10:25
Coffee Break
-
Hydride forming materials
-
29
Microscopic behaviour of hydrogen and hydrides in Atom Probe Tomography of Zirconium
The understanding of Zirconium (Zr) and Hydrogen (H) interactions is a topic of interest in the field of materials science. Zr is known to have a strong affinity with hydrogen, which can lead to the formation of hydrides that can affect the mechanical properties (embrittlement, cracking, etc) of the material [1]. Our studies are carried out on pure Zr analysed by laser-assisted atom probe tomography. This is complex, because the hydrogen detected during the analysis could come from the analysis chamber (parasitic hydrogen) or from the material (hydrogen contained in the material). Our results show the formation of hydrogen species H+, H2+, H3+ and hydrides ZrHx2+. The evolution of the relative abundances of H+, H2+, H3+ depends on the surface field estimated from the Zr3+/Zr2+ ratios, as seen in previous studies on other materials[2]. This is not the case for the hydrides which overlap with the Zr isotopic species. In this contribution we will discuss the quantification of hydrogen and zirconium hydrides, their localization inside the material and their dependence on the field.
[1] J. Bair, M. Asle Zaeem, et M. Tonks, « A review on hydride precipitation in zirconium alloys », J. Nucl. Mater., vol. 466, p. 12‑20, nov. 2015, doi: 10.1016/j.jnucmat.2015.07.014.
[2] L. Rigutti et al., « Surface Microscopy of Atomic and Molecular Hydrogen from Field-Evaporating Semiconductors », J. Phys. Chem. C, vol. 125, no 31, p. 17078‑17087, août 2021, doi: 10.1021/acs.jpcc.1c04778.Speaker: Aissatou Diagne (Univ Rouen Normandie, INSA Rouen Normandie, CNRS, Groupe de Physique des Matériaux UMR 6634, F-76000 Rouen, France) -
30
Investigation of H in Zr and stainless steel tubes from operation in nuclear power reactors
We used APT to investigate Zr-based fuel cladding from boiling water reactor and cold-worked 316L tubes from pressurized water reactor operation.
We carried out voltage pulsing APT on cryo-FIBed Zr cladding (with low success rate); we report on one data set that - contrary to modelling and NanoSIMS experiments - does not show H trapping around an Zr(Fe,Cr)_2-precipitate and discuss how meaningful this result is.
Due to transmutation the 316L steel tube contains H, D and a very small amount of T. In one specimen we found clusters of ions with m/c = 2 Da and those with m/c = 3 Da. We discuss potential explanations for this observation.Speaker: David Mayweg (Chalmers University of Technology)
-
29
-
Structured Discussion
-
12:25
Lunch Break and Departure
-
