Mechano-catalytic depolymerization of plastic waste
Speaker: Prof. Ina Vollmer, Inorganic Chemistry and Catalysis group, Utrecht University
Host: on invitation of Prof. Dierk Raabe
Abstract:
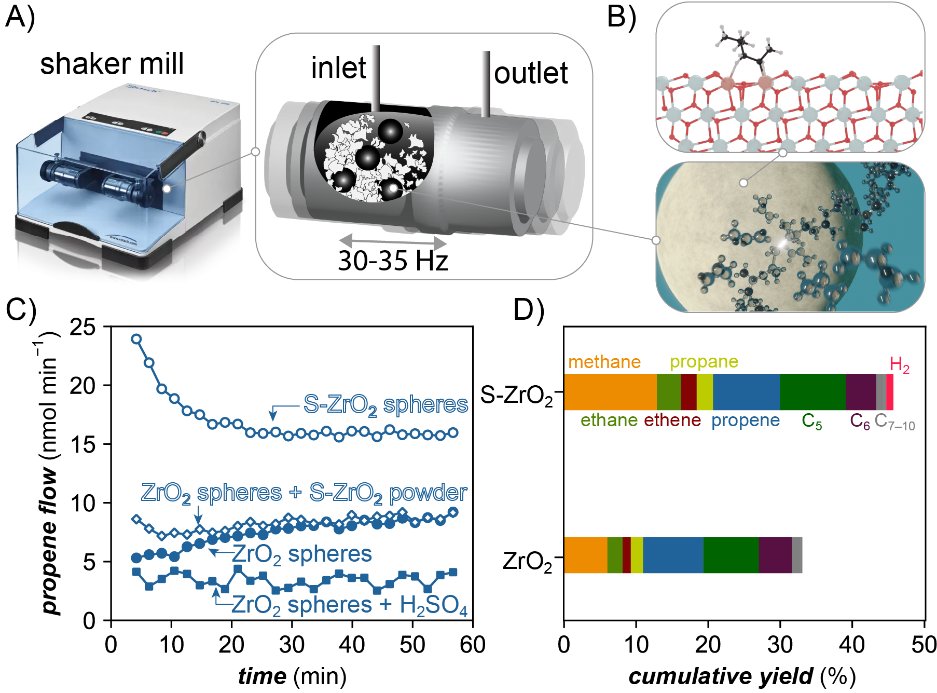
Figure 1. A) Milling container modified with gas in- and outlet to track gaseous product formation. B) Visualization of the mechano-chemical chain cleavage upon ball impact as well as density functional theory optimized structure of a radical interacting with the catalytic surface of the grinding sphere. C) Propene flow during milling of PP with untreated ZrO2 spheres, sulfuric acid treated (S-ZrO2) spheres, untreated spheres with sulfated ZrO2 added as powder, and untreated spheres with sulfuric acid added as liquid. C) Cumulative yields obtained from model PP after 1 h of milling with untreated and S-ZrO2 spheres using optimized conditions.
References
1. Vollmer, I., Jenks, M.F.J., Roelands, M.C.P., et al. Angew. Chem., Int. Ed. 59, 36 (2020).
2. Aydonat, S., Hergesell, A. H., Seitzinger, C. L., Lennarz, R., Chang, G., Sievers, C., Meisner, J., Vollmer, I., & Göstl, R. Polymer Journal (2024).
3. Sakaguchi, M., Sohma, J. J. Polym. Sci., Part B: Polym. Phys. 13, 6 (1975).
4. Rejman, S., Vollmer, I., Werny, M. J., Vogt, E. T. C., Meirer, F., & Weckhuysen, B. M. Chemical Science, 14(37), 10068–10080 (2023).
short bio:
Ina Vollmer is an assistant professor at the Inorganic Chemistry and Catalysis group at Utrecht University. She is looking for pathways to recycle plastics to chemical building blocks using catalysis. Vollmer studied process engineering at Hamburg University of Applied Science, while also obtaining a journalistic degree with a scholarship from the journalistic trainee program of the Konrad-Adenauer foundation. She went on to study chemical engineering at Massachusetts Institute of Technology (graduation 2015). In her doctorate, she investigated the aromatization of methane over zeolite supported metal catalysts using operando spectroscopy with Prof. Freek Kapteijn and Prof. Jorge Gascon (graduated 2019). This included a research stay at King Abdullah University of Science and Technology in Saudi Arabia. After a postdoc on chemical recycling of plastic and natural degradation of plastics to nanoplastics at Utrecht University with Prof. Bert Weckhuysen, Vollmer became an assistant professor in 2021. She teaches sustainable chemistry, polymer chemistry and electron paramagnetic resonance spectroscopy. She is a faculty council member and in the board of the Institute of Sustainable and Circular Chemistry. With her interdisciplinary background in process engineering, chemical engineering, heterogeneous catalysis, spectroscopy, chemical recycling of plastics, polymer chemistry, and mechano-catalysis, Vollmer connects different approaches to come up with new ways to drive chemical conversions. Her idea to convert polyolefins at room temperature in the ball mill via direct catalysis received a Veni and XS grant from the Dutch Research Council (NWO) in 2021 and was patented in 2022.
